One-pot synthesis and antimicrobial efficacy of polymeric schiff base metal complexes derived from o-phenylenediamine and terephthalaldehyde
Keywords:
Schiff base polymeric ligand, Polycondensation, Polymeric schiff base metal complexes, Antimicrobial efficacyAbstract
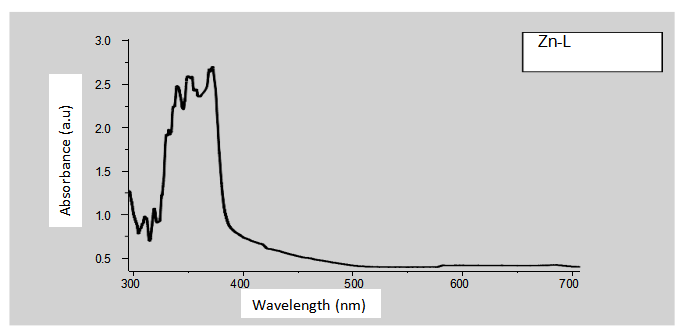
The antimicrobial efficacies of Schiff base Polymeric Ligand (SBPL) and Polymeric Schiff base Metal Complexes (PSBMCs) have been explored. Schiff base Polymeric Ligand (SBPL) and Polymeric Schiff base Metal Complexes (PSBMCs) derived from polycondensation of terephthalaldehyde, o-phenylenediamine and metal ions (M–L, Al3+ , Cu2+ , Zn2+ , Fe3+ ) were synthesized in ethanol at room temperature through one-pot synthesis. The PSBL and PSBMCs synthesized were characterized using spectroscopic techniques: FT-IR (Fourier Transform Infrared) and Uv-vis (Ultraviolet visible). The spectroscopic data revealed that the azomethine functional group is involved in the complexation of metal ions with PSBL forming PSBMCs and geometry of PSBMCs is suggestive to be octahedral. The antibacterial and antifungi efficacies of SBPL and PSBMCs were examined on Staphylococcus aureus, Escherichia coli, Aspergillus niger and Aspergillus flavus employing the agar well diffusion method, Penicillin and omeprazole served as standard drug for antibacterial and antifungal activity respectively. The PBSMCs displayed promising activities in comparison with starting compound (SBPL). The Cu-L was found to be most potent antimicrobial agent against all tested bacterial and fungal.
Published
How to Cite
Issue
Section
Copyright (c) 2025 Wahab Adesina Osunniran, Rukayat Abdulmalik, Sulaiman Habib Adana, Yusuf Oloruntoyin Ayipo

This work is licensed under a Creative Commons Attribution 4.0 International License.







